Introduction
BCL6 co-repressor ( BCOR), a tumor suppressor gene located on chromosome X (locus Xp11.4) is involved in hematopoiesis via non-canonical polycomb repressive complex 1 (PRC1). The prevalence of BCOR mutation in myeloid neoplasms (MN) is approximately 5%, and it is also implicated in clonal hematopoiesis. In the 2022 ELN, WHO, and ICC classifications, BCOR is categorized as a myelodysplasia-related gene. Mutational patterns include frequent co-occurrence with DNMT3A and RUNX1 in AML and mutual exclusivity with NPM1 mutations. Although it has been theorized that BCORMT may cooperate preferentially with certain groups of genes, the prognostic significance of the BCOR co-mutational landscape has yet to be fully studied.
Methods
The total cohort (n = 20149) consists of 624 MN patients (169 primary (p) AML, 136 secondary (s) AML, 84 MDS, 34 MDS/MPN, 66 MPN, and 135 with other MN) from Karmanos Cancer Institute, publicly available metanalytic cohort consisting of various sub-studies (Awada et al., 2021; Kewan et al., 2023), and cohorts from open-access cancer genomics resource cBioPortal and AACR GENIE (v13.1) (Cerami et al., 2012; Gao et al., 2013). Clinical and genomic characteristics were reviewed. Chi-square and T-tests were used for analysis of various parameters as described, and Kaplan-Meier and log-rank methods were used to estimate overall survival (OS). The prevalence of BCORMT, co-occurring mutations, and their outcomes were analyzed based on co-mutational patterns.
Results
A total of 745 (3.7%) patients carried BCORMT. Male: Female ratio was 1.48, with a median age of 68 ys.(IQR 59-75). BCORMTshowed a trend to be most frequent in pAML compared to other MN (54 vs 46%, p=0.33). BCORMT was less associated with abnormal karyotype vs. BCORWT [41 vs. 51%, p=0.0002]. Median variant allele frequency (VAF) was 45% (IQR 22-69), and most mutations were frameshift (41%) followed by nonsense (27%), and missense (22%). Median OS was not different between BCORWT and BCORMT patients [21.9 vs. 19.6 mo., p=0.089] in the entire cohort.
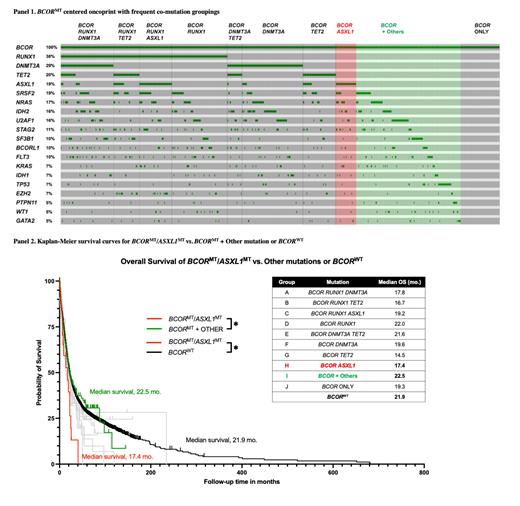
Oncoprint analysis centered around BCOR revealed frequent co-mutations with RUNX1, DNMT3A, TET2 and ASXL1. As a result, the patients were divided into 10 groups (groups A-J), and the OS was analyzed between groups and compared with BCORWT. Patients with BCOR/TET2, BCOR/RUNX1/TET2, and BCOR/ASXL1 (groups G, B, and H resp.) had the lowest median OS with 14.5 mo., 16.7 mo., and 17.4 mo., resp. However, no significant differences in median OS were observed between the compared groups except those involving BCOR/ASXL1 vs. BCOR/Others and BCORWT. BCOR/ASXL1 patients had significantly lower median OS compared to BCOR/Others [17.4 vs. 22.5 mo., p=0.022] and BCORWT [17.4 vs. 21.9 mo., p=0.017].
Further BCORMT and ASXL1MT comutational analysis irrespective of other co-occurring mutations, revealed that in those with BCORMT, ASXL1 co-mutation was seen in 143 (19.2%) patients. BCOR and ASXL1 co-mutation were primarily enriched in primary AML (49%), followed by sAML (25%). Median BCOR VAF was higher than ASXL1 [39 vs. 31%, p<0.0001]. The most associated mutational categories in this subgroup were related to myeloid transcription factors (TF) ( RUNX1, CEBPA; 62%), spliceosome ( SRSF2, U2AF1, SF3B1, ZRSR2; 22%), and DNA methylation family ( DNMT3A, TET2, IDH1/2; 8%). BCORMT/ ASXL1MT/DNA Methylation mutations had poorer median OS compared to BCORMT/ ASXL1MT/Spliceosome mutations [9.2 vs. 17.4 mo., p=0.044]. Whereas BCORMT/ ASXL1MT/Myeloid TF and BCORMT/ ASXL1MT/Spliceosome mutations had no significant survival difference compared to BCORWT, patients with BCORMT/ ASXL1MT/DNA Methylation had worse median OS [9.2 vs. 21.9 mo., p=0.007].
Conclusion
This large cohort study confirms common BCOR co-mutation patterns and reveals prognostic differences in patients with co-occurring ASXL1 mutation. We demonstrated BCORMT with ASXL1MT without concurrent RUNX1MT, DNMT3AMT, and TET2MT had poorer overall survival. Furthermore, patients with BCORMT, ASXL1MT, and concomitant DNA methylation-related gene mutations had worse outcomes compared to those with concomitant spliceosome mutation and BCORWT. Our findings suggest that co-mutational patterns of BCOR may further clarify diagnosis and classification schemes, highlighting potential synergies among subcategories of gene mutations and their prognostic value.
Disclosures
Maciejewski:Novartis: Honoraria, Speakers Bureau; Alexion: Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria; Omeros: Consultancy. Balasubramanian:Karyopharm Therapeutics: Other: Drug supply for research; Kura Oncology: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal